Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
- Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2024;39(2):353-363. Published online January 23, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1809

- 904 View
- 40 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Polyunsaturated fatty acids (PUFAs) reportedly have protective effects on pancreatic β-cells; however, the underlying mechanisms are unknown.
Methods
To investigate the cellular mechanism of PUFA-induced cell protection, mouse insulinoma 6 (MIN6) cells were cultured with palmitic acid (PA) and/or docosahexaenoic acid (DHA), and alterations in cellular signaling and apoptosis were examined.
Results
DHA treatment remarkably repressed caspase-3 cleavage and terminal deoxynucleotidyl transferase-mediated UTP nick end labeling (TUNEL)-positive red dot signals in PA-treated MIN6 cells, with upregulation of autophagy, an increase in microtubule- associated protein 1-light chain 3 (LC3)-II, autophagy-related 5 (Atg5), and decreased p62. Upstream factors involved in autophagy regulation (Beclin-1, unc51 like autophagy activating kinase 1 [ULK1], phosphorylated mammalian target of rapamycin [mTOR], and protein kinase B) were also altered by DHA treatment. DHA specifically induced phosphorylation on S2448 in mTOR; however, phosphorylation on S2481 decreased. The role of G protein-coupled receptor 120 (GPR120) in the effect of DHA was demonstrated using a GPR120 agonist and antagonist. Additional treatment with AH7614, a GPR120 antagonist, significantly attenuated DHA-induced autophagy and protection. Taken together, DHA-induced autophagy activation with protection against PA-induced apoptosis mediated by the GPR120/mTOR axis.
Conclusion
These findings indicate that DHA has therapeutic effects on PA-induced pancreatic β-cells, and that the cellular mechanism of β-cell protection by DHA may be a new research target with potential pharmacotherapeutic implications in β-cell protection.

- Diabetes, obesity and metabolism
- Inhibition of Sodium-Glucose Cotransporter-2 during Serum Deprivation Increases Hepatic Gluconeogenesis via the AMPK/AKT/FOXO Signaling Pathway
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Yu-Mi Lim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2024;39(1):98-108. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1786

- 1,406 View
- 80 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Sodium-dependent glucose cotransporter 2 (SGLT2) mediates glucose reabsorption in the renal proximal tubules, and SGLT2 inhibitors are used as therapeutic agents for treating type 2 diabetes mellitus. This study aimed to elucidate the effects and mechanisms of SGLT2 inhibition on hepatic glucose metabolism in both serum deprivation and serum supplementation states.
Methods
Huh7 cells were treated with the SGLT2 inhibitors empagliflozin and dapagliflozin to examine the effect of SGLT2 on hepatic glucose uptake. To examine the modulation of glucose metabolism by SGLT2 inhibition under serum deprivation and serum supplementation conditions, HepG2 cells were transfected with SGLT2 small interfering RNA (siRNA), cultured in serum-free Dulbecco’s modified Eagle’s medium for 16 hours, and then cultured in media supplemented with or without 10% fetal bovine serum for 8 hours.
Results
SGLT2 inhibitors dose-dependently decreased hepatic glucose uptake. Serum deprivation increased the expression levels of the gluconeogenesis genes peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α), glucose 6-phosphatase (G6pase), and phosphoenolpyruvate carboxykinase (PEPCK), and their expression levels during serum deprivation were further increased in cells transfected with SGLT2 siRNA. SGLT2 inhibition by siRNA during serum deprivation induces nuclear localization of the transcription factor forkhead box class O 1 (FOXO1), decreases nuclear phosphorylated-AKT (p-AKT), and p-FOXO1 protein expression, and increases phosphorylated-adenosine monophosphate-activated protein kinase (p-AMPK) protein expression. However, treatment with the AMPK inhibitor, compound C, reversed the reduction in the protein expression levels of nuclear p- AKT and p-FOXO1 and decreased the protein expression levels of p-AMPK and PEPCK in cells transfected with SGLT2 siRNA during serum deprivation.
Conclusion
These data show that SGLT2 mediates glucose uptake in hepatocytes and that SGLT2 inhibition during serum deprivation increases gluconeogenesis via the AMPK/AKT/FOXO1 signaling pathway.

- Diabetes, obesity and metabolism
- Coronary Artery Calcium Score as a Sensitive Indicator of Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus: A Long-Term Cohort Study
- Dae-Jeong Koo, Mi Yeon Lee, Sun Joon Moon, Hyemi Kwon, Sang Min Lee, Se Eun Park, Cheol-Young Park, Won-Young Lee, Ki Won Oh, Sung Rae Cho, Young-Hoon Jeong, Eun-Jung Rhee
- Endocrinol Metab. 2023;38(5):568-577. Published online October 10, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1770

- 1,552 View
- 113 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Coronary artery calcium score (CACS) has become an important tool for evaluating cardiovascular disease (CVD). This study evaluated the significance of CACS for future CVD through more than 10 years of follow-up in asymptomatic Korean populations with type 2 diabetes mellitus (T2DM) known to have a relatively low CACS burden.
Methods
We enrolled 981 asymptomatic T2DM patients without CVD at baseline who underwent CACS evaluation using multidetector computed tomography between January 2008 and December 2014. They were grouped into five predefined CACS categories based on Agatston scores and followed up by August 2020. The primary endpoint was incident CVD events, including coronary, cerebrovascular, and peripheral arterial disease.
Results
The relative risk of CVD was significantly higher in patients with CACS ≥10, and the significance persisted after adjustment for known confounders. A higher CACS category indicated a higher incidence of future CVD: hazard ratio (95% confidence interval) 4.09 (1.79 to 9.36), 12.00 (5.61 to 25.69), and 38.79 (16.43 to 91.59) for 10≤ CACS <100, 100≤ CACS <400, and CACS ≥400, respectively. During the 12-year follow-up period, the difference in event-free survival more than doubled as the category increased. Patients with CACS below 10 had very low CVD incidence throughout the follow-up. The receiver operating characteristic analysis showed better area under curve when the CACS cutoff was 10 than 100.
Conclusion
CACS can be a sensitive marker of CVD risk. Specifically, CACS above 10 is an indicator of CVD high-risk requiring more intensive medical treatment in Koreans with T2DM.

- Thyroid
- The Current Status of Hyperthyroidism in Korea
- Hyemi Kwon
- Endocrinol Metab. 2023;38(4):392-394. Published online August 25, 2023
- DOI: https://doi.org/10.3803/EnM.2023.401
- 1,243 View
- 89 Download

- Miscellaneous
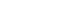
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
- 3,508 View
- 321 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

- Diabetes, Obesity and Metabolism
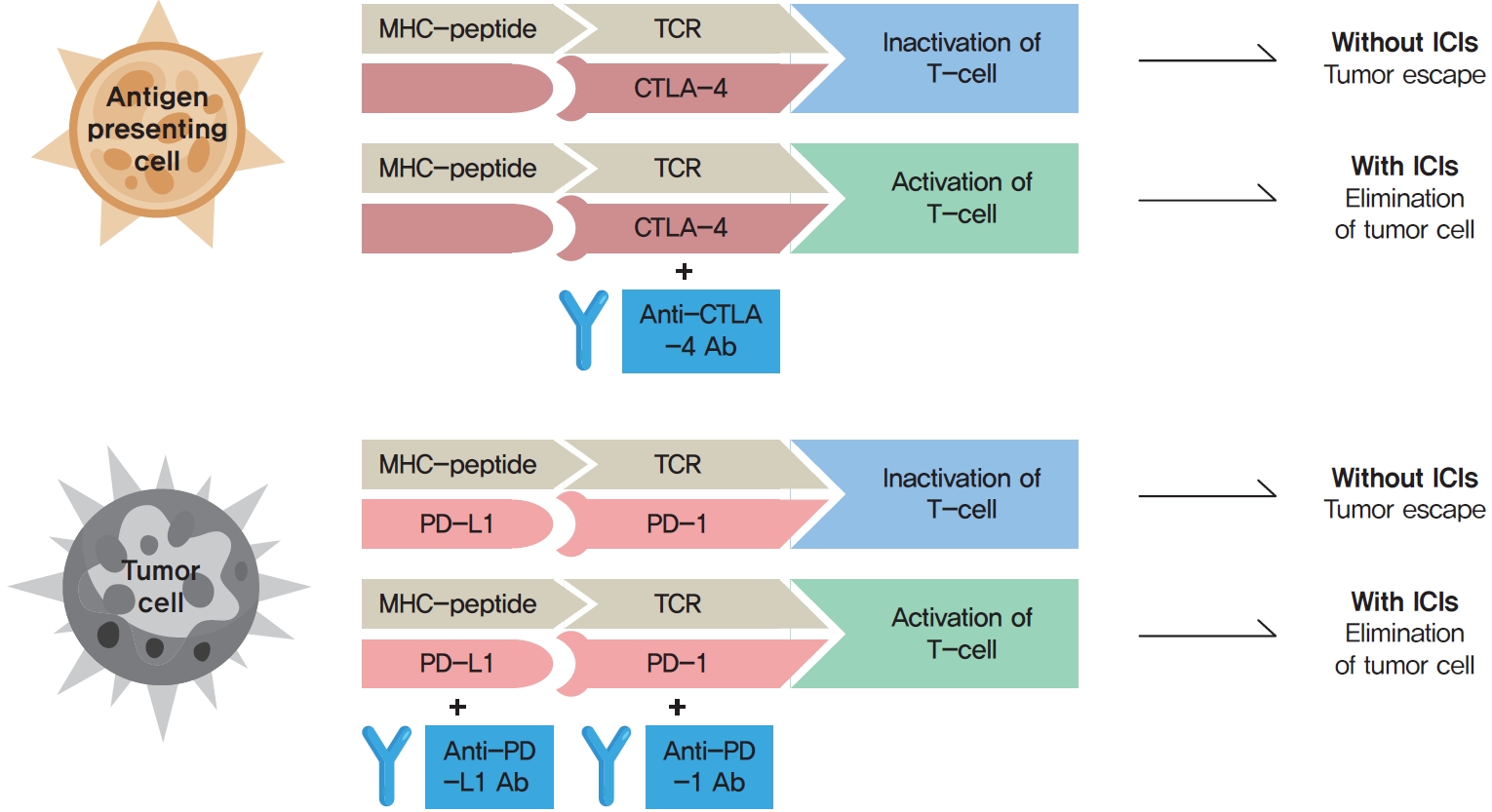
- Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2022;37(1):74-83. Published online February 9, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1293
- 4,900 View
- 235 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Dulaglutide, a long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA), has been shown to reduce body weight and liver fat content in patients with type 2 diabetes. Family with sequence similarity 3 member A (FAM3A) plays a vital role in regulating glucose and lipid metabolism. The aim of this study was to determine the mechanisms by which dulaglutide protects against hepatic steatosis in HepG2 cells treated with palmitic acid (PA).
Methods
HepG2 cells were pretreated with 400 μM PA for 24 hours, followed by treatment with or without 100 nM dulaglutide for 24 hours. Hepatic lipid accumulation was determined using Oil red O staining and triglyceride (TG) assay, and the expression of lipid metabolism-associated factor was analyzed using quantitative real time polymerase chain reaction and Western blotting.
Results
Dulaglutide significantly decreased hepatic lipid accumulation and reduced the expression of genes associated with lipid droplet binding proteins, de novo lipogenesis, and TG synthesis in PA-treated HepG2 cells. Dulaglutide also increased the expression of proteins associated with lipolysis and fatty acid oxidation and FAM3A in PA-treated cells. However, exendin-(9-39), a GLP-1R antagonist, reversed the expression of FAM3A, and fatty acid oxidation-associated factors increased due to dulaglutide. In addition, inhibition of FAM3A by siRNA attenuated the reducing effect of dulaglutide on TG content and its increasing effect on regulation of fatty acid oxidation.
Conclusion
These results suggest that dulaglutide could be used therapeutically for improving nonalcoholic fatty liver disease, and its effect could be mediated in part via upregulation of FAM3A expression through a GLP-1R-dependent pathway. -
Citations
Citations to this article as recorded by- GLP-1/GLP-1RAs: New Options for the Drug Treatment of NAFLD
Haoran Jiang, Linquan Zang
Current Pharmaceutical Design.2024; 30(2): 100. CrossRef - GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives
Riccardo Nevola, Raffaella Epifani, Simona Imbriani, Giovanni Tortorella, Concetta Aprea, Raffaele Galiero, Luca Rinaldi, Raffaele Marfella, Ferdinando Carlo Sasso
International Journal of Molecular Sciences.2023; 24(2): 1703. CrossRef - FAM3A mediates the phenotypic switch of human aortic smooth muscle cells stimulated with oxidised low-density lipoprotein by influencing the PI3K-AKT pathway
Lei Yang, Baoshun Du, Shitao Zhang, Maode Wang
In Vitro Cellular & Developmental Biology - Animal.2023; 59(6): 431. CrossRef - ATP Secretion and Metabolism in Regulating Pancreatic Beta Cell Functions and Hepatic Glycolipid Metabolism
Jing Li, Han Yan, Rui Xiang, Weili Yang, Jingjing Ye, Ruili Yin, Jichun Yang, Yujing Chi
Frontiers in Physiology.2022;[Epub] CrossRef - Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH)
Xiaohan Xu, Kyle L. Poulsen, Lijuan Wu, Shan Liu, Tatsunori Miyata, Qiaoling Song, Qingda Wei, Chenyang Zhao, Chunhua Lin, Jinbo Yang
Signal Transduction and Targeted Therapy.2022;[Epub] CrossRef
- GLP-1/GLP-1RAs: New Options for the Drug Treatment of NAFLD

- Diabetes, Obesity and Metabolism
- Changes in Insulin Resistance Index and the Risk of Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease without Diabetes: Kangbuk Samsung Health Study
- Dae-Jeong Koo, Mi Yeon Lee, Inha Jung, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2021;36(5):1016-1028. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1110

- 4,158 View
- 128 Download
- 5 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Fibrosis is the most important prognostic factor for nonalcoholic fatty liver disease (NAFLD). Insulin resistance plays a key role of fibrosis progression. We evaluated the association between changes in homeostasis model assessment of insulin resistance (HOMA-IR) values and changes in fibrosis status in NAFLD.
Methods
We analyzed the data of 15,728 participants with NAFLD (86% men, mean age 40.5 years) who had no diabetes at baseline and visited our centers for health check-ups both in 2012 and 2016. The participants were classified into four groups according to the degree of change in HOMA-IR values from baseline to the end of follow-up: G1 (<0), G2 (0–0.50), G3 (0.51–1.00), and G4 (>1.00). NAFLD was assessed by ultrasonography, and fibrosis status was evaluated by the NAFLD fibrosis score (NFS) and the aspartate aminotransferase to platelet ratio index (APRI).
Results
After the 4-year follow-up, the multivariable-adjusted odds ratio (OR) for progression of fibrosis probability increased with increasing HOMA-IR values (OR, 2.25; 95% confidence interval [CI], 1.87 to 2.71 for NFS; and OR, 2.55; 95% CI, 2.05 to 3.18 for APRI, G4). This tendency remained consistent throughout the subgroup analyses, except in those for female sex and a body mass index <25 kg/m2. The OR for regression of fibrosis probability decreased with increasing HOMA-IR values (OR, 0.33; 95% CI, 0.25 to 0.43 for NFS, G4).
Conclusion
Changes in HOMA-IR values were associated with changes in fibrosis status in patients with NAFLD without diabetes, which underscores the role of insulin resistance in liver fibrosis. -
Citations
Citations to this article as recorded by- Insulin Resistance/Sensitivity Measures as Screening Indicators of Metabolic-Associated Fatty Liver Disease and Liver Fibrosis
Mohammad E. Khamseh, Mojtaba Malek, Soodeh Jahangiri, Sohrab Nobarani, Azita Hekmatdoost, Marieh Salavatizadeh, Samira Soltanieh, Haleh Chehrehgosha, Hoda Taheri, Zeinab Montazeri, Fereshteh Attaran, Faramarz Ismail-Beigi, Fariba Alaei-Shahmiri
Digestive Diseases and Sciences.2024; 69(4): 1430. CrossRef - Association between nonalcoholic fatty liver disease and left ventricular diastolic dysfunction: A 7-year retrospective cohort study of 3,496 adults using serial echocardiography
Gyuri Kim, Tae Yang Yu, Jae Hwan Jee, Ji Cheol Bae, Mira Kang, Jae Hyeon Kim
Diabetes & Metabolism.2024; : 101534. CrossRef - Factors Associated with Liver Fibrosis in Chinese Patients with Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease
Yu Luo, Cuiyu Wang, Tian Zhang, Xiaoyu He, Jianan Hao, Andong Shen, Hang Zhao, Shuchun Chen, Luping Ren
International Journal of General Medicine.2023; Volume 16: 293. CrossRef - Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study
Ángel Arturo López-González, Bárbara Altisench Jané, Luis Masmiquel Comas, Sebastiana Arroyo Bote, Hilda María González San Miguel, José Ignacio Ramírez Manent
Nutrients.2022; 14(14): 2795. CrossRef - Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease
Jun-Hyuk Lee, Yu-Jin Kwon, Kyongmin Park, Hye Sun Lee, Hoon-Ki Park, Jee Hye Han, Sang Bong Ahn
Nutrients.2022; 14(15): 3039. CrossRef - Machine learning models including insulin resistance indexes for predicting liver stiffness in United States population: Data from NHANES
Kexing Han, Kexuan Tan, Jiapei Shen, Yuting Gu, Zilong Wang, Jiayu He, Luyang Kang, Weijie Sun, Long Gao, Yufeng Gao
Frontiers in Public Health.2022;[Epub] CrossRef - The crosstalk between insulin resistance and nonalcoholic fatty liver disease/metabolic dysfunction-associated fatty liver disease: a culprit or a consequence?
Dae-Jeong Koo, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(4): 132. CrossRef
- Insulin Resistance/Sensitivity Measures as Screening Indicators of Metabolic-Associated Fatty Liver Disease and Liver Fibrosis

- Diabetes, Obesity and Metabolism
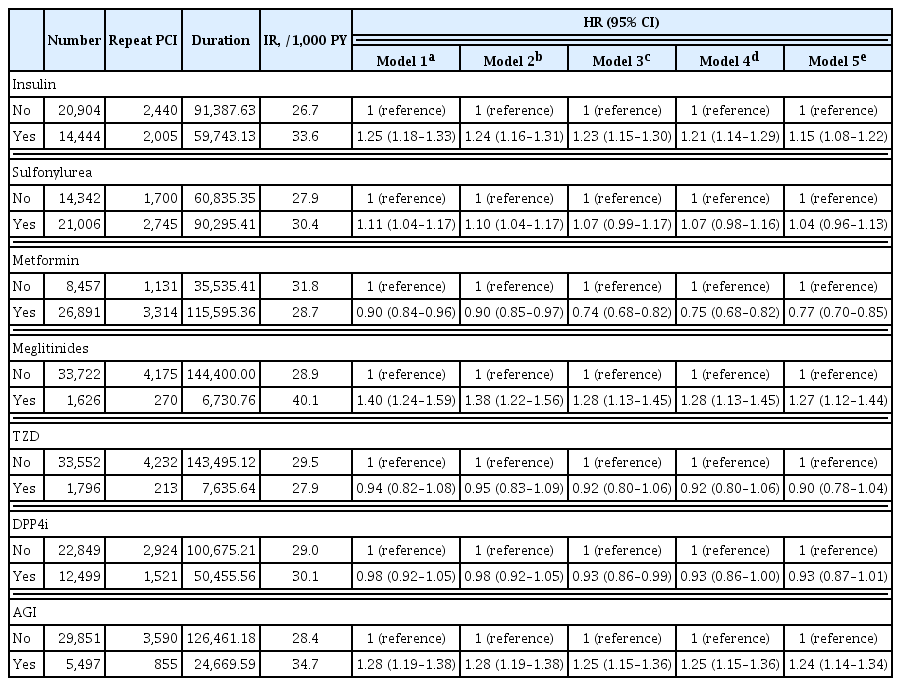
Big Data Articles (National Health Insurance Service Database) - The Effects of Glucose Lowering Agents on the Secondary Prevention of Coronary Artery Disease in Patients with Type 2 Diabetes
- Inha Jung, Hyemi Kwon, Se Eun Park, Kyung-Do Han, Yong-Gyu Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2021;36(5):977-987. Published online October 14, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1046
- 4,025 View
- 175 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Patients with diabetes have a higher risk of requiring repeated percutaneous coronary intervention (PCI) than non-diabetic patients. We aimed to evaluate and compare the effects of anti-diabetic drugs on the secondary prevention of myocardial infarction among type 2 diabetes mellitus patients.
Methods
We analyzed the general health check-up dataset and claims data of the Korean National Health Insurance Service of 199,714 participants (age ≥30 years) who underwent PCIs between 2010 and 2013. Those who underwent additional PCI within 1 year of their first PCI (n=3,325) and those who died within 1 year (n=1,312) were excluded. Patients were classified according to their prescription records for glucose-lowering agents. The primary endpoint was the incidence rate of coronary revascularization.
Results
A total of 35,348 patients were included in the study. Metformin significantly decreased the risk of requiring repeat PCI in all patients (adjusted hazard ratio [aHR], 0.77). In obese patients with body mass index (BMI) ≥25 kg/m2, patients treated with thiazolidinedione (TZD) exhibited a decreased risk of requiring repeat revascularization than those who were not treated with TZD (aHR, 0.77; 95% confidence interval, 0.63 to 0.95). Patients treated with metformin showed a decreased risk of requiring revascularization regardless of their BMI. Insulin, meglitinide, and alpha-glucosidase inhibitor were associated with increased risk of repeated PCI.
Conclusion
The risk of requiring repeat revascularization was lower in diabetic patients treated with metformin and in obese patients treated with TZD. These results suggest that physicians should choose appropriate glucose-lowering agents for the secondary prevention of coronary artery disease. -
Citations
Citations to this article as recorded by- Application of systemic inflammation indices and lipid metabolism-related factors in coronary artery disease
Zhuoyan Zhao, Huan Lian, Yixiang Liu, Lixian Sun, Ying Zhang
Coronary Artery Disease.2023; 34(5): 306. CrossRef - Effect of metformin on adverse outcomes in T2DM patients: Systemic review and meta-analysis of observational studies
Zhicheng Xu, Haidong Zhang, Chenghui Wu, Yuxiang Zheng, Jingzhou Jiang
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Establishment of a Predictive Model for Poor Prognosis of Incomplete Revascularization in Patients with Coronary Heart Disease and Multivessel Disease
Huan Lian, Zhuoyan Zhao, Kelin Ma, Zhenjiang Ding, Lixian Sun, Ying Zhang
Clinical and Applied Thrombosis/Hemostasis.2022; 28: 107602962211392. CrossRef
- Application of systemic inflammation indices and lipid metabolism-related factors in coronary artery disease

- Diabetes, Obesity and Metabolism
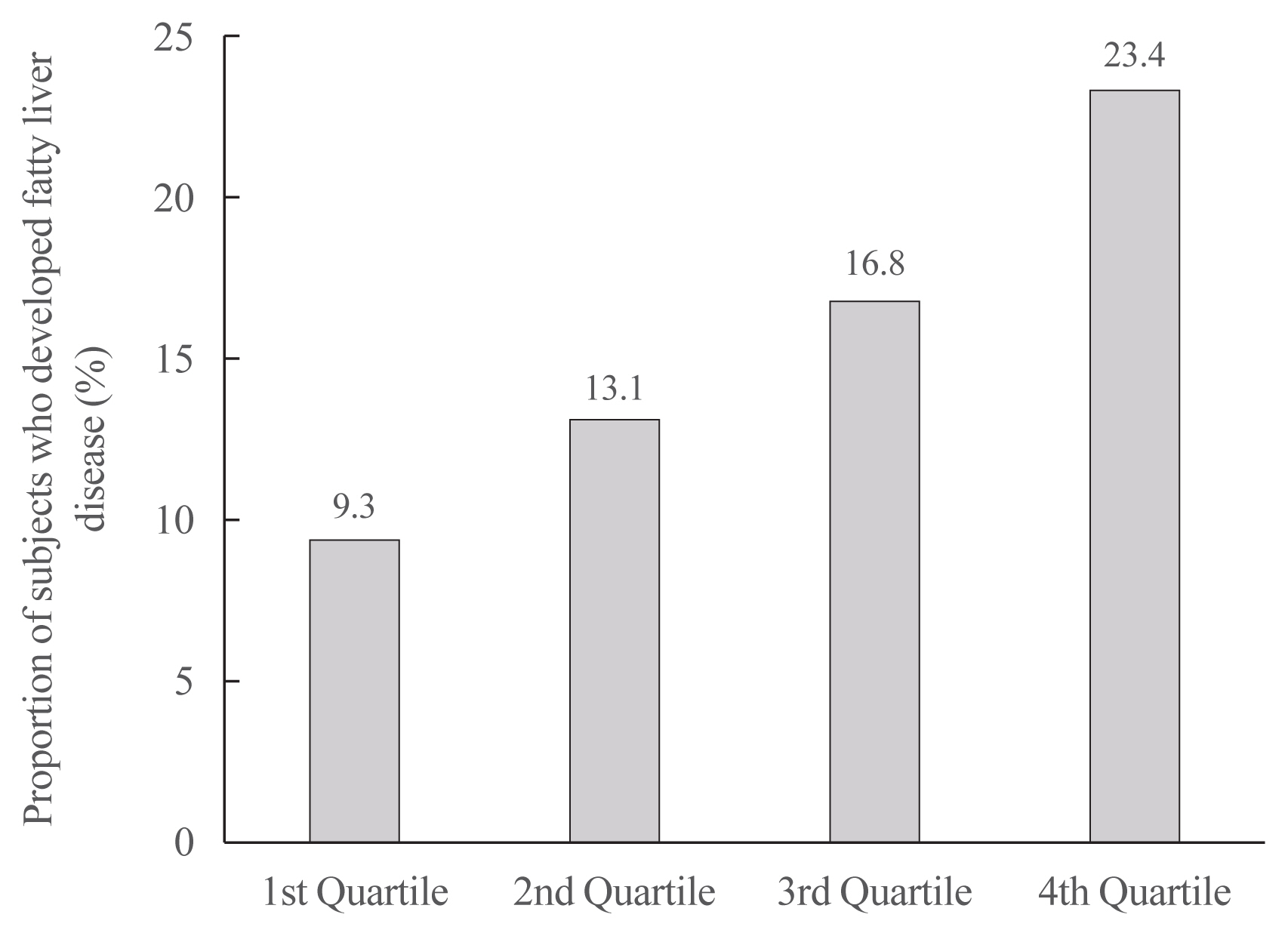
- Increased Risk of Nonalcoholic Fatty Liver Disease in Individuals with High Weight Variability
- Inha Jung, Dae-Jeong Koo, Mi Yeon Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2021;36(4):845-854. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1098
- 4,927 View
- 140 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Weight loss through lifestyle modification is recommended for patients with nonalcoholic fatty liver disease (NAFLD). Recent studies have suggested that repeated loss and gain of weight is associated with worse health outcomes. This study aimed to examine the association between weight variability and the risk of NAFLD in patients without diabetes.
Methods
We examined the health-checkup data of 30,708 participants who had undergone serial examinations between 2010 and 2014. Weight variability was assessed using coefficient of variation and the average successive variability of weight (ASVW), which was defined as the sum of absolute weight changes between successive years over the 5-year period divided by 4. The participants were classified according to the baseline body mass index and weight difference over 4 years.
Results
On dividing the participants into four groups according to ASVW quartile groups, those in the highest quartile showed a significantly increased risk of NAFLD compared to those in the lowest quartile (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.63 to 2.19). Among participants without obesity at baseline, individuals with high ASVW showed increased risk of NAFLD (OR, 1.80; 95% CI, 1.61 to 2.01). Participants with increased weight over 4 years and high ASVW demonstrated higher risk of NAFLD compared to those with stable weight and low ASVW (OR, 4.87; 95% CI, 4.29 to 5.53).
Conclusion
Regardless of participant baseline obesity status, high weight variability was associated with an increased risk of developing NAFLD. Our results suggest that further effort is required to minimize weight fluctuations after achieving a desirable body weight. -
Citations
Citations to this article as recorded by- Changes in Macronutrients during Dieting Lead to Weight Cycling and Metabolic Complications in Mouse Model
Anouk Charlot, Anthony Bringolf, Léa Debrut, Joris Mallard, Anne-Laure Charles, Emilie Crouchet, Delphine Duteil, Bernard Geny, Joffrey Zoll
Nutrients.2024; 16(5): 646. CrossRef - Body weight variability and the risk of liver‐related outcomes in type 2 diabetes and steatotic liver disease: a cohort study
Nathalie C. Leite, Claudia R. L. Cardoso, Cristiane A. Villela‐Nogueira, Gil F. Salles
Obesity.2024;[Epub] CrossRef - Weight variability, physical functioning and incident disability in older adults
Katie J. McMenamin, Tamara B. Harris, Joshua F. Baker
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(4): 1648. CrossRef - Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2022; 37(1): 74. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight
Mohammed Eslam, Hashem B. El-Serag, Sven Francque, Shiv K. Sarin, Lai Wei, Elisabetta Bugianesi, Jacob George
Nature Reviews Gastroenterology & Hepatology.2022; 19(10): 638. CrossRef - Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study
Ángel Arturo López-González, Bárbara Altisench Jané, Luis Masmiquel Comas, Sebastiana Arroyo Bote, Hilda María González San Miguel, José Ignacio Ramírez Manent
Nutrients.2022; 14(14): 2795. CrossRef - Higher Weight Variability Could Bring You a Fatty Liver
Yeoree Yang, Jae-Hyoung Cho
Endocrinology and Metabolism.2021; 36(4): 766. CrossRef - Autonomic Imbalance Increases the Risk for Non-alcoholic Fatty Liver Disease
Inha Jung, Da Young Lee, Mi Yeon Lee, Hyemi Kwon, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Won-Young Lee, Sung-Woo Park, Se Eun Park
Frontiers in Endocrinology.2021;[Epub] CrossRef
- Changes in Macronutrients during Dieting Lead to Weight Cycling and Metabolic Complications in Mouse Model

- Miscellaneous
- COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
- Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim, the Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2021;36(4):757-765. Published online August 17, 2021
- DOI: https://doi.org/10.3803/EnM.2021.404
- 10,369 View
- 419 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Since the first outbreak of coronavirus disease 2019 (COVID-19), ongoing efforts have been made to discover an efficacious vaccine against COVID-19 to combat the pandemic. In most countries, both mRNA and DNA vaccines have been administered, and their side effects have also been reported. The clinical course of COVID-19 and the effects of vaccination against COVID-19 are both influenced by patients’ health status and involve a systemic physiological response. In view of the systemic function of endocrine hormones, endocrine disorders themselves and the therapeutics used to treat them can influence the outcomes of vaccination for COVID-19. However, there are very limited data to support the development of clinical guidelines for patients with specific medical backgrounds based on large clinical trials. In the current severe circumstances of the COVID-19 pandemic, position statements made by clinical specialists are essential to provide appropriate recommendations based on both medical evidence and clinical experiences. As endocrinologists, we would like to present the medical background of COVID-19 vaccination, as well as precautions to prevent the side effects of COVID-19 vaccination in patients with specific endocrine disorders, including adrenal insufficiency, diabetes mellitus, osteoporosis, autoimmune thyroid disease, hypogonadism, and pituitary disorders.
-
Citations
Citations to this article as recorded by- COVID-19 mRNA vaccine may trigger subacute thyroiditis
Mehmet Sözen, Ömercan Topaloğlu, Berrin Çetinarslan, Alev Selek, Zeynep Cantürk, Emre Gezer, Damla Köksalan, Taner Bayraktaroğlu
Human Vaccines & Immunotherapeutics.2024; 17(12): 5120. CrossRef - The role of co-morbidities in the development of an AEFI after COVID-19 vaccination in a large prospective cohort with patient-reported outcomes in the Netherlands
C. Ouaddouh, J.W. Duijster, T. Lieber, F.P.A.M. van Hunsel
Expert Opinion on Drug Safety.2024; 23(3): 323. CrossRef - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
Hyeyeon Moon, Sunghwan Suh, Mi Kyoung Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Prior immunization status of COVID-19 patients and disease severity: A multicenter retrospective cohort study assessing the different types of immunity
Javaria Aslam, Faisal Shahzad Khan, Muhammad Talha Haris, Hewad Hewadmal, Maryam Khalid, Mohammad Y. Alshahrani, Qurrat-ul-ain Aslam, Irrum Aneela, Urooj Zafar
Vaccine.2023; 41(2): 598. CrossRef - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(2): 253. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Inactivated SARS-CoV-2 vaccination does not disturb the clinical course of Graves’ disease: An observational cohort study
Shichen Xu, Huixin Yu, Xian Cheng, Jing Wu, Jiandong Bao, Li Zhang
Vaccine.2023; 41(38): 5648. CrossRef - Adrenal Crisis Associated With COVID-19 Vaccination in Patients With Adrenal Insufficiency
Yukako Kurematsu, Takako Mohri, Sadanori Okada, Yutaka Takahashi
JCEM Case Reports.2023;[Epub] CrossRef - Adverse Events Associated with COVID-19 Vaccination in Adolescents with Endocrinological Disorders: A Cross-Sectional Study
İbrahim Mert Erbaş, İrem Ceren Erbaş, Gözde Akın Kağızmanlı, Kübra Yüksek Acinikli, Özge Besci, Korcan Demir, Ece Böber, Nurşen Belet, Ayhan Abacı
Journal of Clinical Research in Pediatric Endocrinology.2023; 15(3): 248. CrossRef - Neue Aspekte der Glukokortikoidsubstitution bei Nebennierenrindeninsuffizienz
Tina Kienitz, Gesine Meyer
Der Internist.2022; 63(1): 12. CrossRef - Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence
Rimesh Pal, Ameya Joshi, Sanjay K. Bhadada, Mainak Banerjee, Suresh Vaikkakara, Satinath Mukhopadhyay
Endocrine Practice.2022; 28(4): 425. CrossRef - Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study
Xi Xiong, Carlos King Ho Wong, Ivan Chi Ho Au, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Kristy Tsz Kwan Lau, Chi Ho Lee, Yu Cho Woo, David Tak Wai Lui, Ian Chi Kei Wong
Thyroid.2022; 32(5): 505. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism
Nikolina Markovic, Anila Faizan, Chirag Boradia, Sridhar Nambi
AACE Clinical Case Reports.2022; 8(4): 171. CrossRef - The Effect of Inactivated SARS-CoV-2 Vaccines on TRAB in Graves’ Disease
LingHong Huang, ZhengRong Jiang, JingXiong Zhou, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Osteoporosis in Patients With Respiratory Diseases
Yue Ma, Shui Qiu, Renyi Zhou
Frontiers in Physiology.2022;[Epub] CrossRef - Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Ach Taieb, El Euch Mounira
Vaccines.2022; 10(12): 2004. CrossRef - Forty Years Together, New Leap Forward! The 40th Anniversary of the Korean Endocrine Society
Jong Chul Won, Ki-Hyun Baek
Endocrinology and Metabolism.2022; 37(6): 851. CrossRef - No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine
Tania Pilli, Cristina Dalmiglio, Gilda Dalmazio, Alfonso Sagnella, Raffaella Forleo, Lucia Brilli, Fabio Maino, Cristina Ciuoli, Maria Grazia Castagna
European Journal of Endocrinology.2022; 187(1): K7. CrossRef - Diabetes and COVID-19 Vaccination
Hae Dong Choi, Jun Sung Moon
The Journal of Korean Diabetes.2021; 22(4): 221. CrossRef
- COVID-19 mRNA vaccine may trigger subacute thyroiditis

- Endocrine Research
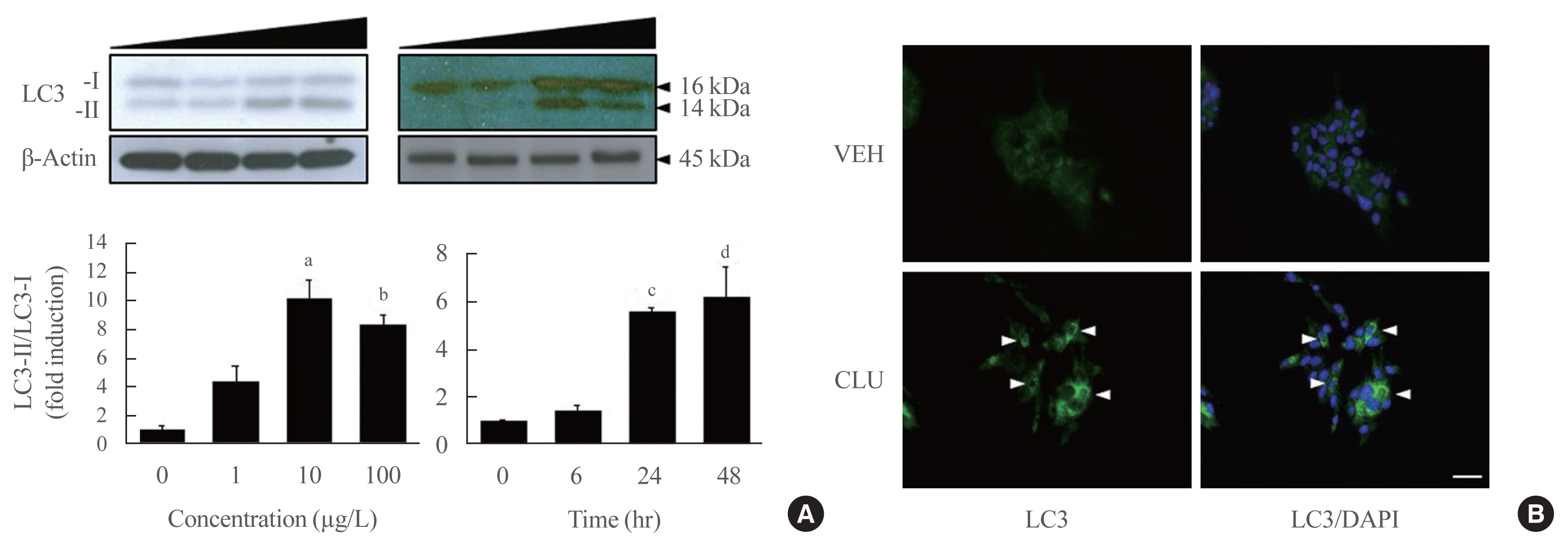
- Clusterin Protects Lipotoxicity-Induced Apoptosis via Upregulation of Autophagy in Insulin-Secreting Cells
- Seok-Woo Hong, Jinmi Lee, Min Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2020;35(4):943-953. Published online December 2, 2020
- DOI: https://doi.org/10.3803/EnM.2020.768
- 5,669 View
- 135 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There is a great need to discover factors that could protect pancreatic β-cells from apoptosis and thus prevent diabetes mellitus. Clusterin (CLU), a chaperone protein, plays an important role in cell protection in numerous cells and is involved in various cellular mechanisms, including autophagy. In the present study, we investigated the protective role of CLU through autophagy regulation in pancreatic β-cells.
Methods
To identify the protective role of CLU, mouse insulinoma 6 (MIN6) cells were incubated with CLU and/or free fatty acid (FFA) palmitate, and cellular apoptosis and autophagy were examined.
Results
Treatment with CLU remarkably upregulated microtubule-associated protein 1-light chain 3 (LC3)-II conversion in a doseand time-dependent manner with a significant increase in the autophagy-related 3 (Atg3) gene expression level, which is a mediator of LC3-II conversion. Moreover, co-immunoprecipitation and fluorescence microscopy experiments showed that the molecular interaction of LC3 with Atg3 and p62 was markedly increased by CLU. Stimulation of LC3-II conversion by CLU persisted in lipotoxic conditions, and FFA-induced apoptosis and dysfunction were simultaneously improved by CLU treatment. Finally, inhibition of LC3-II conversion by Atg3 gene knockdown markedly attenuated the cytoprotective effect of CLU.
Conclusion
Taken together, these findings suggest that CLU protects pancreatic β-cells against lipotoxicity-induced apoptosis via autophagy stimulation mediated by facilitating LC3-II conversion. Thus, CLU has therapeutic effects on FFA-induced pancreatic β-cell dysfunction. -
Citations
Citations to this article as recorded by- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes
Alexandra Coomans de Brachène, Corentin Scoubeau, Anyïshai E. Musuaya, Jose Maria Costa-Junior, Angela Castela, Julie Carpentier, Vitalie Faoro, Malgorzata Klass, Miriam Cnop, Decio L. Eizirik
Diabetologia.2023; 66(3): 450. CrossRef - Apolipoprotein J Attenuates Vascular Restenosis by Promoting Autophagy and Inhibiting the Proliferation and Migration of Vascular Smooth Muscle Cells
Ning Yang, Bo Dong, Yanqiu Song, Yang Li, Lu Kou, Qin Qin
Journal of Cardiovascular Translational Research.2022; 15(5): 1086. CrossRef - Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
Seok-Woo Hong, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 57. CrossRef - Co-regulators of autophagy and the cell cycle in HFD − As treated mice
Marzieh Zeinvand-Lorestani, Mohammad Javad Khodayar, Ali Teimoori, Najmaldin Saki, Akram Ahangarpour, Ali Ranjbar, Hamed Zeinvand-Lorestani
Journal of Trace Elements and Minerals.2022; 2: 100018. CrossRef - Targeting pancreatic β cells for diabetes treatment
Chirag Jain, Ansarullah, Sara Bilekova, Heiko Lickert
Nature Metabolism.2022; 4(9): 1097. CrossRef - Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies
Safia Costes, Gyslaine Bertrand, Magalie A. Ravier
International Journal of Molecular Sciences.2021; 22(10): 5303. CrossRef
- Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes

- Clinical Study
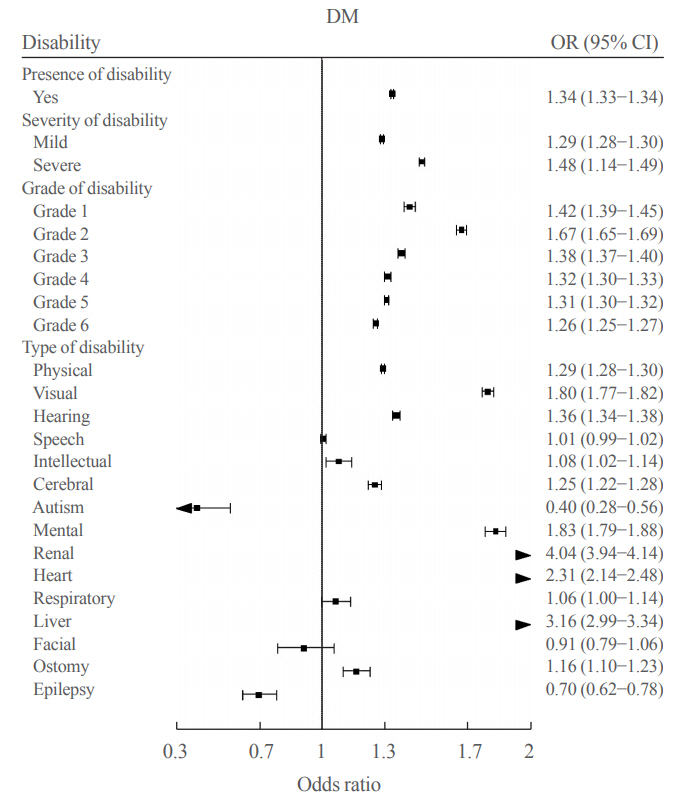
- The Prevalence and Risk of Type 2 Diabetes in Adults with Disabilities in Korea
- Inha Jung, Hyemi Kwon, Se Eun Park, Kyung-Do Han, Yong-Gyu Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2020;35(3):552-561. Published online July 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.653
- 8,105 View
- 188 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
People with disabilities are at risk of secondary conditions such as diabetes. The aim of this study was to evaluate the prevalence and risk of type 2 diabetes in South Korea, especially among people with all types of disabilities.
Methods
We conducted a cross-sectional study using data from the Korean National Health Insurance Service, with two disabilityfree controls matched for each participant with disabilities by age and sex. Information regarding the type, severity and grade of disabilities was obtained based on the National Disability Registry. Diagnosis of type 2 diabetes was defined according to the following criteria: presence of International Classification of Diseases, Tenth Revision, Clinical Modification codes E11, E12, E13, or E14 and claims for at least one oral anti-diabetic agent or insulin at baseline, or fasting glucose level ≥126 mg/dL.
Results
We included 1,297,806 participants with disabilities and 2,943,719 control. Out of 4,241,525 participants, 841,990 (19.9%) were diagnosed with diabetes. The prevalence of diabetes was higher in the disability group compared with individuals without disabilities (23.1% vs. 18.4%). The odds of having diabetes was higher in the disability group compared with the control group (adjusted odds ratio, 1.34; 95% confidence interval, 1.33 to 1.34). The results showed higher prevalence of diabetes in the mildly disabled group (23.2%) than in the severely disabled group (22.7%).
Conclusion
The prevalence and risk of diabetes were higher in people with disabilities compared with the general population. Physicians and public health authorities should focus on people with disabilities for proper diabetes management. -
Citations
Citations to this article as recorded by- Widening disparities in the national prevalence of diabetes mellitus for people with disabilities in South Korea
I. Hwang, S.Y. Kim, Y.Y. Kim, J.H. Park
Public Health.2024; 226: 173. CrossRef - Bipolar disorder and the risk of cardiometabolic diseases, heart failure, and all-cause mortality: a population-based matched cohort study in South Korea
You-Bin Lee, Hyewon Kim, Jungkuk Lee, Dongwoo Kang, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim, Hong Jin Jeon, Kyu Yeon Hur
Scientific Reports.2024;[Epub] CrossRef - Psychotic Disorders and the Risk of Type 2 Diabetes Mellitus, Atherosclerotic Cardiovascular Diseases, and All-Cause Mortality: A Population-Based Matched Cohort Study
You-Bin Lee, Hyewon Kim, Jungkuk Lee, Dongwoo Kang, Gyuri Kim, Sang-Man Jin, Jae Hyeon Kim, Hong Jin Jeon, Kyu Yeon Hur
Diabetes & Metabolism Journal.2024; 48(1): 122. CrossRef - Pathways linking health literacy to self-care in diabetic patients with physical disabilities: A moderated mediation model
Hye Jin Nam, Ju Young Yoon, Wen-Jun Tu
PLOS ONE.2024; 19(3): e0299971. CrossRef - Dysphagia Requiring Medical Attention in Parkinson’s Disease: A Korean Population-Based Study
Seungwoo Cha, Won Kee Chang, Hee-Mun Cho, Kyungdo Han, Nam-Jong Paik, Sohyun Kwon, Won-Seok Kim
Journal of Korean Medical Science.2023;[Epub] CrossRef - Disparities in diabetes-related avoidable hospitalization among diabetes patients with disability using a nationwide cohort study
Hin Moi Youn, Dong-Woo Choi, Sung-In Jang, Eun-Cheol Park
Scientific Reports.2022;[Epub] CrossRef - Disability type–specific mortality patterns and life expectancy among disabled people in South Korea using 10-year combined data between 2008 and 2017
Jinwook Bahk, Hee-Yeon Kang, Young-Ho Khang
Preventive Medicine Reports.2022; 29: 101958. CrossRef - Cholecystectomy reduces the risk of myocardial and cerebral infarction in patients with gallstone-related infection
Seon Mee Park, Hyun Jung Kim, Tae Uk Kang, Heather Swan, Hyeong Sik Ahn
Scientific Reports.2022;[Epub] CrossRef - Nationwide trends in the incidence of tuberculosis among people with disabilities in Korea:
a nationwide serial cross-sectional study
Jinsoo Min, So Young Kim, Jong Eun Park, Yeon Yong Kim, Jong Hyock Park
Epidemiology and Health.2022; 44: e2022098. CrossRef - Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus
Mee Kyoung Kim, Kyungdo Han, Eun Sil Koh, Oak-Kee Hong, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2021; 175: 108799. CrossRef - Diabetes in People with Disabilities: a Call for Action
Inha Jung, Eun-Jung Rhee, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2021; 3(4): 82. CrossRef
- Widening disparities in the national prevalence of diabetes mellitus for people with disabilities in South Korea

- Clinical Study
- Serum Adiponectin and Progranulin Level in Patients with Benign Thyroid Nodule or Papillary Thyroid Cancer
- Hyemi Kwon, Se Eun Park, Ji-Sup Yun, Cheol-Young Park
- Endocrinol Metab. 2020;35(2):396-406. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.396

- 5,643 View
- 108 Download
- 11 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Obesity is associated with thyroid cancer risk. Adiponectin has insulin-sensitizing and anti-inflammatory effects, while progranulin is associated with inflammation and tumorigenesis. We investigated serum adiponectin and progranulin levels in patients with benign thyroid nodule (benign group) and papillary thyroid cancer (PTC; PTC group). The associations between these levels and the clinicopathological features of PTC were evaluated.
Methods
We included 157 patients who underwent thyroid surgery (17% of benign and 83% of PTC group). Clinicopathological features including size, lymph node metastasis, extrathyroidal extension (ETE), multifocality, American Thyroid Association risk stratification were evaluated.
Results
The age was 42.0 years, and 69% were female. Serum adiponectin and progranulin levels were 6.3 μg/mL and 101.5 ng/mL in the benign group and 5.4 μg/mL and 106.1 ng/mL in the PTC group, respectively (P=0.6 and P=0.4, respectively). Serum adiponectin levels showed no significant differences according to clinicopathological features of PTC. The proportions of patients with primary tumor size >1 cm were 3%, 5%, 8%, and 8% according to serum progranulin level quartiles, respectively (P=0.03). The proportions of patients with microscopic/gross ETE were 8%/0%, 9%/1%, 11%/1%, and 11%/2% according to serum progranulin level quartiles, respectively. Median serum progranulin level was significantly higher in patients with PTC >1 cm than in patients with papillary thyroid microcarcinoma (P=0.04, 115.3 ng/mL and 104.7 ng/mL, respectively).
Conclusion
Serum adiponectin and progranulin levels showed no significant difference between benign and PTC groups. Increased serum progranulin levels were significantly associated with PTC >1 cm and microscopic and gross ETE. -
Citations
Citations to this article as recorded by- Exploring the logic and conducting a comprehensive evaluation of AdipoRon-based adiponectin replacement therapy against hormone-related cancers—a systematic review
Lucas Fornari Laurindo, Andreline Franchi Sosin, Caroline Barbalho Lamas, Ricardo de Alvares Goulart, Jesselina Francisco dos Santos Haber, Claudia Rucco Penteado Detregiachi, Sandra Maria Barbalho
Naunyn-Schmiedeberg's Archives of Pharmacology.2024; 397(4): 2067. CrossRef - Adiponectin Inhibits the Progression of Obesity-Associated Papillary Thyroid Carcinoma Through Autophagy
Changlin Li, Jiao Zhang, Gianlorenzo Dionigi, Nan Liang, Haixia Guan, Hui Sun
Endocrinology.2024;[Epub] CrossRef - Progranulin Oncogenic Network in Solid Tumors
Elisa Ventura, Giacomo Ducci, Reyes Benot Dominguez, Valentina Ruggiero, Antonino Belfiore, Elena Sacco, Marco Vanoni, Renato V. Iozzo, Antonio Giordano, Andrea Morrione
Cancers.2023; 15(6): 1706. CrossRef - Obesity and thyroid cancer risk
Lauren C. Burrage, Donald S.A. McLeod, Susan J. Jordan
Current Opinion in Endocrinology, Diabetes & Obesity.2023; 30(5): 244. CrossRef - Progranulin promoted the proliferation, metastasis, and suppressed apoptosis via JAK2-STAT3/4 signaling pathway in papillary thyroid carcinoma
Yanxu Dong, Hao Tan, Lidong Wang, Zhen Liu
Cancer Cell International.2023;[Epub] CrossRef - Obesity and Thyroid Cancer Risk: An Update
Fabiana Franchini, Giuseppe Palatucci, Annamaria Colao, Paola Ungaro, Paolo Emidio Macchia, Immacolata Cristina Nettore
International Journal of Environmental Research and Public Health.2022; 19(3): 1116. CrossRef - Obesity and Overweight Are Associated with Minimal Extrathyroidal Extension, Multifocality and Bilaterality of Papillary Thyroid Cancer
Krzysztof Kaliszewski, Dorota Diakowska, Marta Rzeszutko, Jerzy Rudnicki
Journal of Clinical Medicine.2021; 10(5): 970. CrossRef - Adiponectin and Thyroid Cancer: Insight into the Association between Adiponectin and Obesity
Yuanyuan Zhou, Ying Yang, Taicheng Zhou, Bai Li, Zhanjian Wang
Aging and disease.2021; 12(2): 597. CrossRef
- Exploring the logic and conducting a comprehensive evaluation of AdipoRon-based adiponectin replacement therapy against hormone-related cancers—a systematic review

- Clinical Study
- Visceral-to-Subcutaneous Abdominal Fat Ratio Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis
- Chan-Hee Jung, Eun-Jung Rhee, Hyemi Kwon, Yoosoo Chang, Seungho Ryu, Won-Young Lee
- Endocrinol Metab. 2020;35(1):165-176. Published online March 19, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.1.165

- 6,531 View
- 137 Download
- 26 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background We evaluated the association of visceral-to-subcutaneous fat ratio (VSR) with nonalcoholic fatty liver disease (NAFLD) and advanced fibrosis degree based on noninvasive serum fibrosis markers in the general population with NAFLD.
Methods This is a cross-sectional study, in 7,465 Korean adults who underwent health screening examinations. NAFLD was defined as fatty liver detected on ultrasonography, and visceral and subcutaneous abdominal fat was measured using computed tomography. We predicted fibrosis based on the fibrosis-4 (FIB-4) score and aspartate aminotransferase-to-platelet ratio index (APRI) and categorized the risk for advanced fibrosis as low, indeterminate, or high.
Results The multivariable-adjusted prevalence ratios for indeterminate to high risk of advanced fibrosis based on FIB-4, determined by comparing the second, third, and fourth quartiles with the first quartile of VSR, were 3.38 (95% confidence interval [CI], 0.64 to 17.97), 9.41 (95% CI, 1.97 to 45.01), and 19.34 (95% CI, 4.06 to 92.18), respectively. The multivariable-adjusted prevalence ratios for intermediate to high degree of fibrosis according to APRI also increased across VSR quartiles (5.04 [95% CI, 2.65 to 9.59], 7.51 [95% CI, 3.91 to 14.42], and 19.55 [95% CI, 9.97 to 38.34], respectively). High VSR was more strongly associated with the prevalence of NAFLD in nonobese subjects than in obese subjects, and the associations between VSR and intermediate to high probability of advanced fibrosis in NAFLD were stronger in obese subjects than in nonobese subjects.
Conclusion High VSR values predicted increased NAFLD risk and advanced fibrosis risk with NAFLD, and the predictive value of VSR for indeterminate to high risk of advanced fibrosis was higher in obese subjects than in nonobese subjects.
-
Citations
Citations to this article as recorded by- Opportunistic Extraction of Quantitative CT Biomarkers: Turning the Incidental Into Prognostic Information
Mohammad Nazri Md Shah, Raja Rizal Azman, Wai Yee Chan, Kwan Hoong Ng
Canadian Association of Radiologists Journal.2024; 75(1): 92. CrossRef - Positive Association Between the Chinese Visceral Adiposity Index and Nonalcoholic Fatty Liver Disease in Lean Adults
Shuxia Shen, Hangkai Huang, Jinghua Wang, Zexi Tang, Chao Shen, Chengfu Xu
Digestive Diseases and Sciences.2023; 68(2): 656. CrossRef - Association between Sarcopenic Obesity Status and Nonalcoholic Fatty Liver Disease and Fibrosis
Wolhwa Song, Sung Hwan Yoo, Jinsun Jang, Su Jung Baik, Byoung Kwon Lee, Hyun Woong Lee, Jong Suk Park
Gut and Liver.2023; 17(1): 130. CrossRef - Using hyperhomocysteinemia and body composition to predict the risk of non-alcoholic fatty liver disease in healthcare workers
Xiaoyan Hao, Honghai He, Liyuan Tao, Peng Wang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Visceral and subcutaneous fat, muscle mass, and liver volume as noninvasive predictors of the progress of non-alcoholic fatty liver disease
Omar M. Mahmoud, Gehad Abd Elaziz Mahmoud, Haisam Atta, Wael A. Abbas, Hanan M. Ahmed, Mohamed A. A. Abozaid
Egyptian Journal of Radiology and Nuclear Medicine.2023;[Epub] CrossRef - Relationship between metabolic associated fatty liver disease and body fat ratio, visceral fat area, and resting metabolic rate estimated by bioelectrical impedance analysis
Deng-Hua He, Yong-Zhan Zhang, Liang Xu, Jia-Jia Pei, Ying Zhang, Zhong-Fang Yan
World Chinese Journal of Digestology.2023; 31(2): 56. CrossRef - Poor glycaemic control and ectopic fat deposition mediates the increased risk of non-alcoholic steatohepatitis in high-risk populations with type 2 diabetes: Insights from Bayesian-network modelling
T. Waddell, A. Namburete, P. Duckworth, A. Fichera, A. Telford, H. Thomaides-Brears, D. J. Cuthbertson, M. Brady
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Subcutaneous Fat Obesity in a High Body Mass Index Donor Is Not a Contraindication to Living Donor Hepatectomy
Hirak Pahari, Amey Sonavane, Amruth Raj, Anup Kumar Agrawal, Ambreen Sawant, Deepak Kumar Gupta, Amit Gharat, Vikram Raut, Sorabh Kapoor
Case Reports in Hepatology.2023; 2023: 1. CrossRef - Comparison of cardiometabolic risk factors between obese and non-obese patients with nonalcoholic fatty liver disease
Zahra Yari, Danial Fotros, Azita Hekmatdoost
Scientific Reports.2023;[Epub] CrossRef - Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity
Hong-Kyu Kim, Sung-Jin Bae, Min Jung Lee, Eun Hee Kim, Hana Park, Hwi Seung Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee, Jaewon Choe
Clinical and Molecular Hepatology.2023; 29(4): 987. CrossRef - Visceral Adipose Tissue Inflammation and Radiographic Visceral-to-Subcutaneous Adipose Tissue Ratio in Patients with Cirrhosis
Nghiem B. Ha, Soo-Jin Cho, Yara Mohamad, Dorothea Kent, Grace Jun, Randi Wong, Vivek Swarnakar, Shezhang Lin, Jacquelyn J. Maher, Jennifer C. Lai
Digestive Diseases and Sciences.2022; 67(7): 3436. CrossRef - The Influence of Obesity and Metabolic Health on Vascular Health
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(1): 1. CrossRef - The effect of combined exercises on the plasma levels of retinol-binding protein 4 and its relationship with insulin resistance and hepatic fat content in postmenopausal women with nonalcoholic fatty liver disease
Masoumeh NOROUZPOUR, Sayyed M. MARANDI, Mohsen GHANBARZADEH, Abbasali ZARE MAIVAN
The Journal of Sports Medicine and Physical Fitness.2022;[Epub] CrossRef - The Perirenal Fat Thickness Was Associated with Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus
Yuxian Yang, Shuting Li, Yuechao Xu, Jing Ke, Dong Zhao
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 1505. CrossRef - Visceral adiposity is an independent risk factor for high intra-operative blood loss during living-donor liver transplantation; could preoperative rehabilitation and nutritional therapy mitigate that risk?
Mahmoud Macshut, Toshimi Kaido, Siyuan Yao, Yosuke Miyachi, Mohamed Sharshar, Sena Iwamura, Masaaki Hirata, Hisaya Shirai, Naoko Kamo, Shintaro Yagi, Shinji Uemoto
Clinical Nutrition.2021; 40(3): 956. CrossRef - A review of non‐alcoholic fatty liver disease in non‐obese and lean individuals
Mitra Ahadi, Kasra Molooghi, Negin Masoudifar, Ali Beheshti Namdar, Hassan Vossoughinia, Mohammadreza Farzanehfar
Journal of Gastroenterology and Hepatology.2021; 36(6): 1497. CrossRef - Quantification of abdominal fat from computed tomography using deep learning and its association with electronic health records in an academic biobank
Matthew T MacLean, Qasim Jehangir, Marijana Vujkovic, Yi-An Ko, Harold Litt, Arijitt Borthakur, Hersh Sagreiya, Mark Rosen, David A Mankoff, Mitchell D Schnall, Haochang Shou, Julio Chirinos, Scott M Damrauer, Drew A Torigian, Rotonya Carr, Daniel J Rader
Journal of the American Medical Informatics Association.2021; 28(6): 1178. CrossRef - Hepatic Steatosis in Patients With Single Ventricle and a Fontan Circulation
David A. Katz, Daniel Peck, Adam M. Lubert, Mathias Possner, Faizeen Zafar, Andrew T. Trout, Joseph J. Palermo, Nadeem Anwar, Jonathan R. Dillman, Adam W. Powell, Stavra A. Xanthakos, Alexander R. Opotowsky, Gruschen Veldtman, Tarek Alsaied
Journal of the American Heart Association.2021;[Epub] CrossRef - Superficial vs Deep Subcutaneous Adipose Tissue: Sex-Specific Associations With Hepatic Steatosis and Metabolic Traits
Tessa Brand, Inge Christina Lamberta van den Munckhof, Marinette van der Graaf, Kiki Schraa, Helena Maria Dekker, Leonardus Antonius Bernardus Joosten, Mihai Gheorghe Netea, Niels Peter Riksen, Jacqueline de Graaf, Joseph Henricus Wilhelmus Rutten
The Journal of Clinical Endocrinology & Metabolism.2021; 106(10): e3881. CrossRef - Baseline homeostasis model assessment of insulin resistance associated with fibrosis progression in patients with nonalcoholic fatty liver disease without diabetes: A cohort study
Dae-Jeong Koo, Mi Yeon Lee, Inha Jung, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee, Ming-Lung Yu
PLOS ONE.2021; 16(8): e0255535. CrossRef - Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non‐alcoholic fatty liver disease assessed by magnetic resonance imaging
Anne Flint, Grit Andersen, Paul Hockings, Lars Johansson, Anni Morsing, Mads Sundby Palle, Thomas Vogl, Rohit Loomba, Leona Plum‐Mörschel
Alimentary Pharmacology & Therapeutics.2021; 54(9): 1150. CrossRef - Effects of IL-33 on 3T3-L1 cells and obese mice models induced by a high-fat diet
Yue Kai, Jingtao Gao, Hu Liu, Yubing Wang, Chenrui Tian, Sheng Guo, Ling He, Min Li, Zhongwei Tian, Xiangfeng Song
International Immunopharmacology.2021; 101: 108209. CrossRef - Lipid Accumulation Product as an Index for Visceral Obesity and Cardiovascular Risk among a Sample of Obese Egyptian Women
Nayera E. Hassan, Sahar A. El-Masry, Gamila S. M. El-Saeed, Mohamed S. El Hussieny
Open Access Macedonian Journal of Medical Sciences.2021; 9(B): 1229. CrossRef - Combined Effects of Dyslipidemia and High Adiposity on the Estimated Glomerular Filtration Rate in a Middle-Aged Chinese Population
Xichang Wang, Haoyu Wang, Jiashu Li, Xiaotong Gao, Yutong Han, Weiping Teng, Zhongyan Shan, Yaxin Lai
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4513. CrossRef - Determination of “indeterminate score” measurements in lean nonalcoholic fatty liver disease patients from western Saudi Arabia
Yasir Mohammed Khayyat
World Journal of Hepatology.2021; 13(12): 2150. CrossRef - Utility of Liver Function Tests and Fatty Liver Index to Categorize Metabolic Phenotypes in a Mediterranean Population
Dariusz Narankiewicz, Josefina Ruiz-Nava, Veronica Buonaiuto, María Isabel Ruiz-Moreno, María Dolores López-Carmona, Luis Miguel Pérez-Belmonte, Ricardo Gómez-Huelgas, María Rosa Bernal-López
International Journal of Environmental Research and Public Health.2020; 17(10): 3518. CrossRef
- Opportunistic Extraction of Quantitative CT Biomarkers: Turning the Incidental Into Prognostic Information

- Thyroid
- Letter: Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country (Endocrinol Metab 2018;33:466-72, Carmen Castillo et al.)
- Hyemi Kwon
- Endocrinol Metab. 2019;34(1):93-94. Published online March 21, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.1.93
- 4,061 View
- 59 Download
- 1 Web of Science


 KES
KES

 First
First Prev
Prev